The Working Groups
Digestion and Dispersion Working Group - led by Professor Colin Pouton (Professor of Pharmaceutical Biology at the University of Monash, Melbourne Australia):
The participating laboratories in this Group has focused on the performance on digestion of different types of formulations (Types I, II, IIIA IIIB, IV) according to the current LFCS classification. These formulations have been incorporated with a series of model poorly water-soluble drugs and have been assessed systematically across a range of digestion conditions.
Over 3 years, significant progress has been made and several milestones reached, some of which include:
- Development of standard set of digestion test conditions and protocol to be used within the consortium
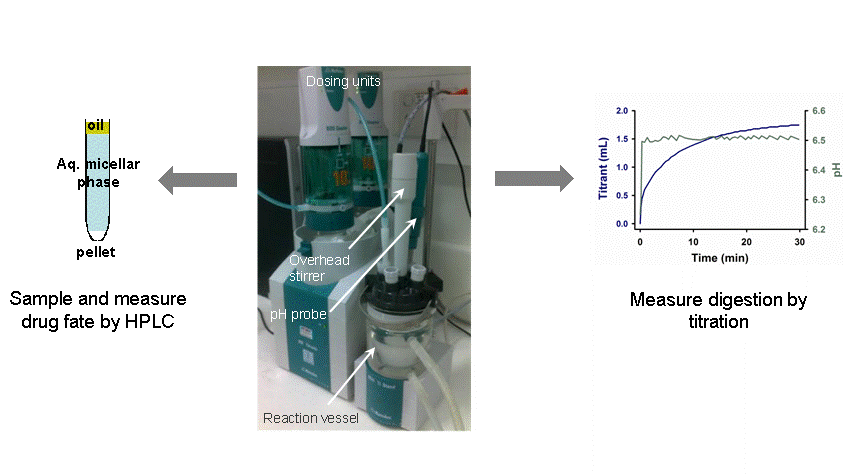
- Metrohm pH-stat apparatus was selected as the standard digestion equipment as it generated reproducible testing conditions
- Consistent in vitro digestion data was obtained in two laboratories at the first attempt
The process of processing samples removed during digestion was simplified so that a greater number of samples could be removed per test
- The effect of several variables on LBDDS performance during digestion was assessed including bile salt concentration, pancreatin activities, Ca2+ effect, drug saturation level and dilution
- A new parameter, namely the maximum supersaturation ratio (SRM), shown to strongly determine with the fate of the several different drugs as a LBDDS is digested
- Performance of semi-solid LBDDS were also assessed under LFCS digestion test conditions
- 11 posters at AAPS
- Three published manuscripts and an additional five planned over the next 12 months (see LFCS Publications)
In year 3, the digestion group also turned their focus toward standardizing methods to evaluate the in vitro dispersion of LBDDS. Dispersion tests are generally less challenging to the performance of LBDDS compared to digestion tests, but provide the opportunity to rapidly screen-out formulations that poorly disperse or those that cannot maintain the drug in solution following simple dilution. Formulations that exhibit good performance on dispersion are progressed onto digestion tests. In the dispersion work program, the performance of five formulations containing four different drugs has been assessed across a series of dispersion test conditions. Preliminary dispersion findings will be presented at the 2013 AAPS Annual Meeting.
In addition, the dispersion/digestion group has begun to perform a series of in vivo studies to support the extensive in vitro data-sets acquired during the first three years of the LFCS Consortium. The first in vivo study has been completed, and the results of this study will be presented during at the 2013 AAPS Annual Meeting. Three remaining dog studies have been scheduled and are underway.
Taking together the results of in vitro dispersion and digestion, and the in vivo studies, the dispersion/digestion working group is generating results that benefit the scientific community by providing guidance on how to test LBDDS, proposing standard test conditions, identifying key factors that impact performance and proposing new LBDDS performance criteria. Therefore, with continuing efforts from the LFCS Consortium, the field is increasingly better placed to develop robust LBDDS.
Current Digestion Working Group Participants:
Communication Working Group - led by Hassan Benameur, Ph.D. (Senior Director, Global Pharmaceutical Sciences, Capsugel)
The Communication Working Group has set the following objectives:
- Establish a scientific database that will serve as an evidence-based reference source for the formulation of poorly water-soluble drugs using LBDDS
- Create and maintain the LFCS Consortium website and communications policy
- Organize SAC and Working Group meeting timetable and logistics
- Organize symposia and a calendar of events
Current Communication Working Group participants are: