History behind the LFCS Consortium
2005
The idea to create the LFCS Consortium began during the AAPS meeting in Nashville, Tennessee, on the initiative of Hassan Benameur, Ph.D., (Senior Director, Global Pharmaceutical Sciences, Capsugel), and Professor Colin Pouton (Professor of Pharmaceutical Biology at Monash University, Australia).
2006
An initial tour of interested parties took place across the US in November 2006. Discussions were held with several Pharmaceutical companies in the US and Europe.
2007
A meeting of interested European companies was hosted in Barcelona, Spain in June 2007. Key Japanese players have been made aware of the initiative.
During the 2007 AAPS meeting in San Diego, California, a meeting including 22 participants was held. A proposal was put forth by Prof. Colin Pouton to develop the LFCS through Monash University where laboratory work could be funded by a consortium of companies from the pharmaceutical and supplier industries. The structure of the Consortium was considered and some relevant aspects were discussed. It was further proposed that funding could be managed through the Gattefossé foundation.
After this meeting, Prof. Jennifer Dressman (Goethe Universität, Frankfurt am Main) expressed her interest to Hassan and Colin to actively participate in the establishment of LFCS.
2008
In June 2008, a Memorandum of Understanding, establishing the Terms and Conditions governing the LFCS Consortium in its current structure was drafted.
During the 2008 AAPS Annual Meeting in Atlanta, Georgia, the first annual LFCS meeting was held where the MOU was discussed, finalized and circulated between the interested parties.
2009
Throughout 2009, Working Group members and Full and Associate Members were identified and Working Group programs were drafted.
As a consequence, an LFCS Consortium Agreement was next circulated, negotiated and finalized in December 2009.
The Gattefossé Foundation, (under the aegis of Fondation Rhone Alpes Futur) acts as a Trustee for the LFCS Consortium.
In late 2009 the initial planning and design of the LFCS Consortium website was commenced.
2010
Public Release of the LFCS Consortium Website by CoAcS
The first year of LFCS Consortium activity commenced with 3 Working Groups:
- Dispersion: The objective of this group was to focus on the dispersion performance of different types of formulations according to Pouton’s LFCS Classification and to study key parameters and types of equipment for dispersion/precipitation.
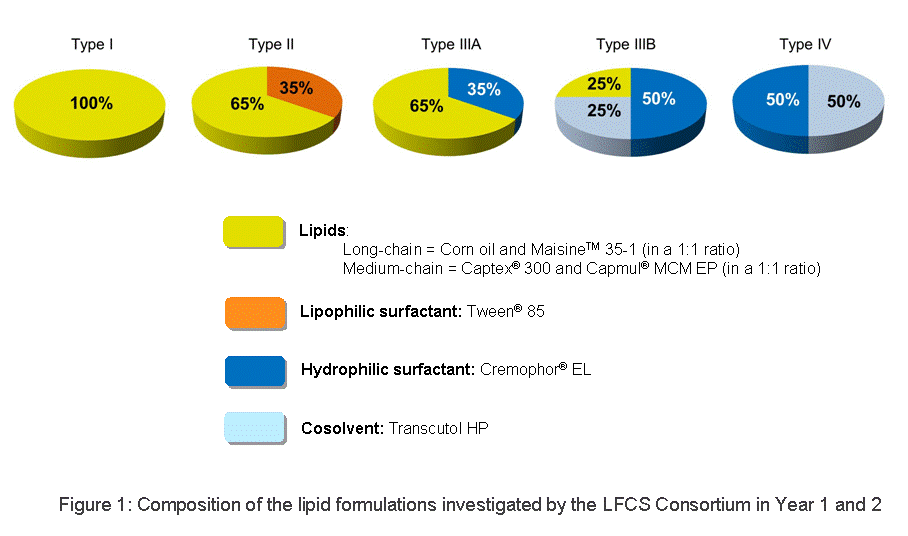
- Digestion: This group began to study representative formulations (Types I, II, IIIa, IIIb, IV), see Figure 1 below, of poorly soluble drugs and vary key parameters to assess the likelihood and importance of drug precipitation during digestion. Suitable equipment was adopted to improve the reproducibility of in vitro digestion testing. The results generated will be used to guide the development of recommended methods, and subsequently SOPs, to study the fate of lipid-based drug delivery systems. It is intended that the recommended methods will be validated by multiple academic and industrial laboratories.
- Communication: This group took ownership of website establishment and update, meeting organization, communication inside and outside the Consortium.
The LFCS Consortium consisted of 7 leading academic institutions and 8 industrial partners from Australia, Europe, and the US.
2011
January 2011: At the First Annual Meeting in Barcelona the Digestion Group presented key findings of the work performed during Year 1. Discussions were held about year 2 activity. The Dispersion Group declined to participate at the meeting and did not present an acceptable program for Year 2.
The decision was taken to focus more on key performance criteria for digestion during the beginning of 2011. A new organizational structure will be proposed in 2011 to enable the research on methods for assessment of performance of lipid based formulations during dispersion to recommence, with the goal to resume laboratory-based activities later in 2011.
In vitro digestion studies continued during Year 2 of the LFCS Consortium with the primary focus of expanding into a wider drug space while still using the broad range of lipid formulations investigated in year 1.
October: Four LFCS Consortium posters summarizing Year 1 experimental work was presented at the AAPS Annual Meeting in Washington DC.
2012
January: The Second Annual Meeting was held in Montpellier where experimental work in Year 2 was discussed and work-plans for Year 3 were finalized.
September: The first paper from the LFCS Consortium was published in the Journal of Pharmaceutical Sciences (see the LFCS Publications section)
October: The second paper from the LFCS Consortium was published in the journal Molecular Pharmaceutics (see the LFCS Publications section). In addition, four LFCS Consortium posters summarizing Year 2 experimental work was presented at the AAPS Annual Meeting in Chicago.

|
|
| LFCS Consortium Paper 1 |
LFCS Consortium Paper 2 |
2013
February: The Third Annual Meeting was held in Strasbourg where experimental work in Year 3 was presented. Final year 3 activities and the results of the first in vivo dog study were also discussed.

Members of the LFCS Consortium at the 2013 annual meeting held in Strasbourg
April: The third publication from the LFCS Consortium was accepted for publication in the journal Pharmaceutical Research (see the LFCS Publications section)
June: Three LFCS abstracts submitted to the AAPS were accepted for poster presentation at the AAPS Annual Meeting in San Antonio.
Present position: In vivo dog studies are currently being performed. The results of these studies and feedback from current members of the consortium are both being used to define experimental proposals for future LFCS Consortium activities.

LFCS Consortium paper 3