Welcome to the LFCS Consortium
Unlike most oral drug delivery systems, lipid-based drug delivery systems
(LBDDS) have the potential to increase the oral bioavailability of poorly
water-soluble drugs via several different mechanisms that include:
(i)
Bypassing the dissolution step by delivering the
drug in a pre-dissolved form and avoidance of re-precipitation from this
pre-dissolved state
(ii)
Increasing drug solubilization in the intestinal milieu
directly through formulation components formed upon digestion of formulation
excipients and indirectly by recruiting natural solubilizers (e.g. bile salts
and phospholipid)
(iii)
Increasing intestinal drug permeability through inhibition of P-gp and other
efflux transporters
(iv)
Decreasing first-pass metabolism of the drug through recruitment of
intestinal lymphatic processes.
All of the above processes have the potential to enhance bioavailability in the
fasted state to decrease the high risk of a food-effect and establish the
desired reproducible pharmacokinetic profile of orally administered drugs.
Furthermore, lipids are physiologically well tolerated and absorbed. As a
result, an increasing number of academic and industrial formulation groups
concentrate their efforts in this field of research and development, and an
increasing number of lipid formulated products have reached the market in recent
years.
Within a challenging pharmaceutical development environment, the sharing of
knowledge and expertise, and combination of research efforts is an effective way
to advance science and address common technical problems. The Lipid Formulation
Classification System (LFCS) Consortium aims to use collective resources to
solve generic problems that help development of novel and optimized therapies
for healthcare professionals and the patients they serve.
The LFCS Consortium is a non-profit organization that sponsors and conducts
research on lipid based drug delivery systems (LBDDS) for the oral
administration of poorly water-soluble drugs.
Inspired by Professor Colin Pouton’s Lipid Formulation Classification System
(first published in 2000 and in modified form in 2006; see Table 1 below), with
the joint leadership of Capsugel, the LFCS Consortium has been established as a
small, yet growing scientific community of industrial and academic professionals
with a unique focus and expertise covering all scientific and technical aspects
that relate to LBDDS, from formulation and in vitro characterization, to
enhancement of in vivo bioavailability of poorly soluble compounds
The primary objective of the LFCS Consortium is to develop guidelines that
rationalize and accelerate the development of promising drug candidates,
specifically through:
(i)
The identification of LBDDS key performance
criteria
(ii)
The validation and publication of universal
Standard Operating Procedures to assess performance
(iii)
Initiating an appropriate dialogue with
pharmaceutical regulatory bodies (EMEA, FDA) to establish approved guidelines
for evaluating the performance of LBDDS.
Today, industrial partners of the LFCS Consortium sponsor research programs
conducted by multinational University members under the leadership and direction
of the LFCS Consortium Scientific Advisory Committee. The primary research aims
are to develop standardized in vitro methods to assess the performance of LBDDS
during dispersion and digestion and to use these methods to identify the key
factors that determine LBDDS performance.
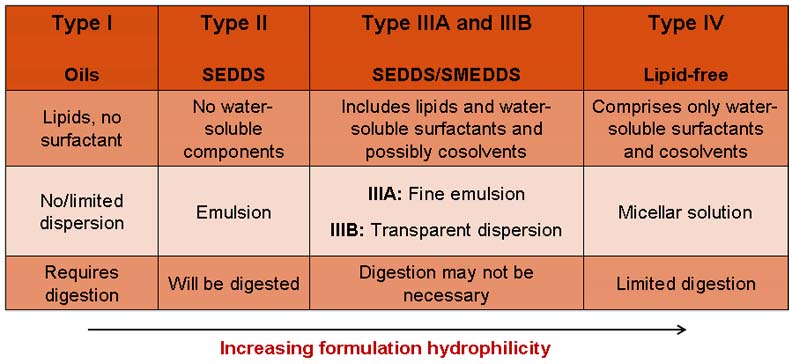
Table1: The Lipid Formulation Classification System
Today, industrial partners at Full and Associate Membership levels sponsor research programs conducted by multinational University members under the leadership and direction of the LFCS Consortium Scientific Advisory Committee. The research aims to develop in vitro methods to assess the performance of LBDDS during dispersion and digestion.